Medical Devices Vigilance Market Scope: Growth, Share, Value, Size, and Analysis
Medical Devices Vigilance Market Scope: Growth, Share, Value, Size, and Analysis
Blog Article
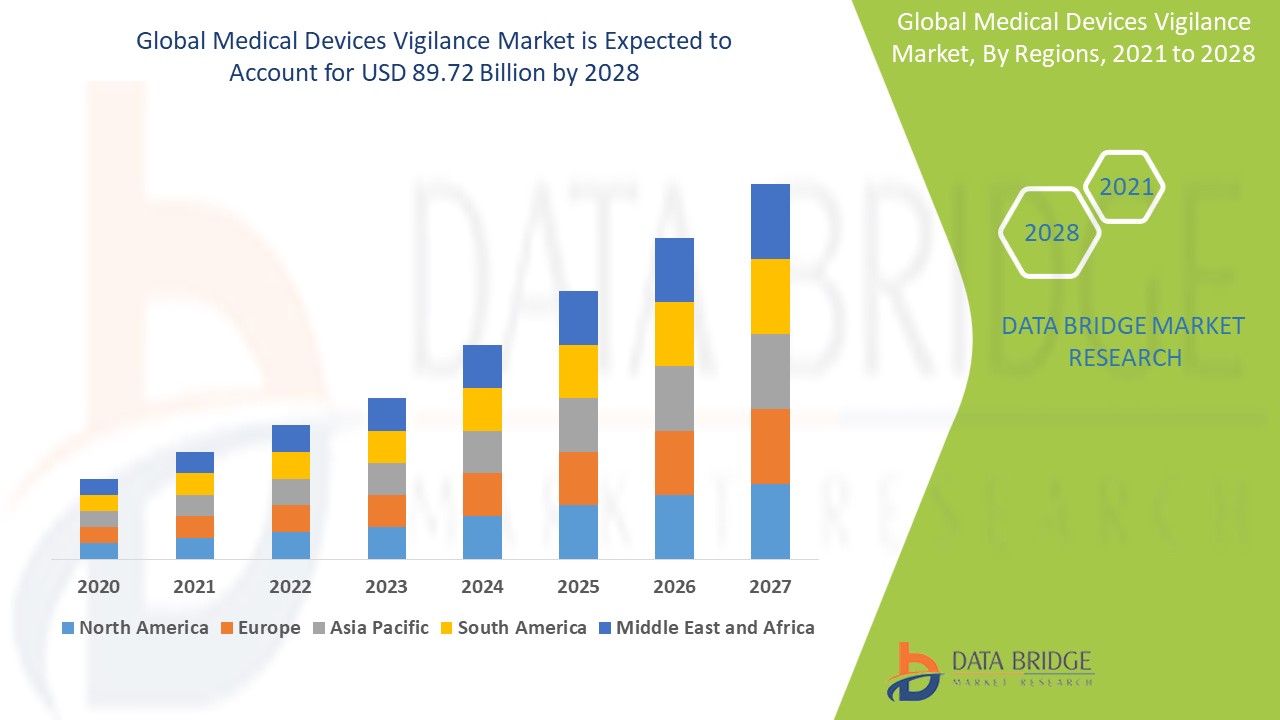
"Medical Devices Vigilance Market Size And Forecast by 2028
According to Data Bridge Market Research Medical devices vigilance market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses the market to account to USD 89.72 billion by 2028 growing at a CAGR of 6.89% in the above-mentioned forecast period.
Innovation remains at the core of Healthcare Device Compliance Market success, driving growth and customer engagement. Medical Devices Vigilance Market continuously invests in research and development to stay ahead of industry changes. By leveraging advanced technology, Medical Equipment Safety Monitoring Market enhances its solutions to meet consumer expectations. The strategic approach of Medical Devices Vigilance Market ensures that new products and services remain competitive. Medical Devices Vigilance Market remains a pioneer, delivering top-tier solutions with innovative strategies.
Post-Market Surveillance for Medical Devices Market plays a crucial role in shaping global market trends through its dynamic approach. The influence of Medical Devices Vigilance Market extends across industries, inspiring growth and development. Companies look to Medical Device Risk Management Market as a benchmark for success, recognizing the brand’s leadership. By staying updated with consumer needs, Medical Devices Vigilance Market maintains its strong position. The adaptability of Health Tech Regulatory Compliance Market ensures continued influence in shaping industry patterns.
Our comprehensive Medical Devices Vigilance Market report is ready with the latest trends, growth opportunities, and strategic analysis. https://www.databridgemarketresearch.com/reports/global-medical-devices-vigilance-market
**Segments**
- **By Device Type:** The medical devices vigilance market can be segmented based on the type of devices, which includes active medical devices, non-active medical devices, and implantable medical devices. Active medical devices such as pacemakers and infusion pumps require specific vigilance due to their direct interaction with patients. Non-active medical devices like bandages and braces also fall under this category, while implantable medical devices like artificial joints and pacemakers require continuous monitoring for any safety concerns.
- **By Application:** Segmentation by application includes monitoring medical devices for adverse incidents, conducting risk assessments, and implementing corrective actions. Adverse event monitoring involves tracking device-related incidents reported by healthcare professionals or patients. Risk assessments are essential to evaluate the severity and likelihood of potential hazards associated with medical devices. Corrective actions involve implementing measures to address safety issues identified during monitoring and risk assessment processes.
- **By End-User:** The medical devices vigilance market can also be segmented based on end-users, such as hospitals, ambulatory surgical centers, clinics, and diagnostic laboratories. Hospitals are significant users of medical devices and play a crucial role in reporting adverse incidents related to device usage. Ambulatory surgical centers and clinics also utilize various medical devices, requiring vigilance to ensure patient safety. Diagnostic laboratories that use medical devices for testing purposes are also part of the end-user segment.
**Market Players**
- **Medtronic:** A leading player in the global medical devices vigilance market, Medtronic provides a range of medical devices across different therapeutic areas. The company focuses on ensuring the safety and performance of its products through robust vigilance programs and continuous monitoring of device-related incidents.
- **Johnson & Johnson:** With a diverse portfolio of medical devices, Johnson & Johnson is actively involved in implementing vigilance measures to maintain the quality and safety of its products. The company emphasizes proactive risk assessment and timely corrective actions to address any safety concerns.
- **Abbott Laboratories:** Abbott Laboratories is a key player in the medical devices vigilance market, known for its innovative medical devices and advanced monitoring systems. The company places high importance on risk management and vigilance activities to enhance patient safety and product quality.
- **Becton, Dickinson and Company (BD):** BD is a prominent player in the medical devices sector, offering a wide range of devices for healthcare professionals. The company's vigilance strategies focus on prompt incident reporting, thorough risk analysis, and effective communication to stakeholders to ensure the continuous improvement of device safety.
The global medical devices vigilance market is characterized by the presence of several leading players that prioritize patient safety and regulatory compliance to maintain market competitiveness and trust among consumers. Through comprehensive vigilance programs and continuous innovation, these market players contribute significantly to enhancing healthcare quality and device safety.
https://www.databridgemarketresearch.com/reports/global-medical-devices-vigilance-Market The global medical devices vigilance market holds significant potential for growth and innovation as the healthcare industry continues to prioritize patient safety and regulatory compliance. Market players like Medtronic, Johnson & Johnson, Abbott Laboratories, and Becton, Dickinson and Company (BD) are key contributors to the market's evolution through their robust vigilance programs and commitment to enhancing device safety. These industry leaders leverage advanced technologies, proactive risk assessment strategies, and effective corrective actions to address safety concerns and ensure the quality of their medical devices. By focusing on continuous monitoring, thorough risk analysis, and timely incident reporting, these companies maintain a competitive edge in the market while building trust among consumers and healthcare professionals.
Moreover, the segmentation of the medical devices vigilance market based on device type, application, and end-user provides valuable insights into the diverse requirements and challenges within the industry. Active medical devices, non-active medical devices, and implantable medical devices each present specific vigilance needs that require tailored monitoring and risk assessment approaches to ensure patient safety. Monitoring medical devices for adverse incidents, conducting risk assessments, and implementing corrective actions are essential components of effective vigilance practices that help mitigate risks and enhance the overall quality of healthcare delivery.
The market dynamics of the medical devices vigilance sector are influenced by regulatory frameworks, technological advancements, and the increasing demand for innovative healthcare solutions globally. As healthcare providers and regulatory bodies focus on enhancing patient outcomes and reducing adverse events related to medical devices, the vigilance market is expected to witness steady growth and investment opportunities. Market players will need to adapt to evolving regulatory requirements, implement proactive vigilance strategies, and prioritize collaboration with healthcare stakeholders to address emerging challenges and drive sustainable growth in the sector.
In conclusion, the global medical devices vigilance market is poised for continued expansion and transformation driven by the commitment of leading market players to ensuring patient safety and product quality. By embracing vigilance best practices, implementing robust risk management strategies, and fostering innovation in device safety, these companies are instrumental in shaping the future of healthcare delivery and improving outcomes for patients worldwide. As the industry evolves, collaboration, innovation, and regulatory compliance will be crucial factors in sustaining growth and driving excellence in medical device vigilance.**Segments**
Global Medical Devices Vigilance Market, By Delivery Mode:
- On-Demand/Cloud Based (SAAS) Delivery Mode
- On-Premises Delivery Mode
Application:
- Diagnostic
- Therapeutic
- Surgical
- Research
End-User:
- Clinical Research Organizations (CROs)
- Original Equipment Manufacturers (OEMs)
- Business Process Outsourcing (BPO)
Country:
- U.S.
- copyright
- Mexico
- Germany
- Italy
- U.K.
- France
- Spain
- Netherlands
- Belgium
- Switzerland
- Turkey
- Russia
- Rest of Europe
- Japan
- China
- India
- South Korea
- Australia
- Singapore
- Malaysia
- Thailand
- Indonesia
- Philippines
- Rest of Asia-Pacific
- Brazil
- Argentina
- Rest of South America
- South Africa
- Saudi Arabia
- U.A.E
- Egypt
- Israel
- Rest of Middle East and Africa
Industry Trends and Forecast to 2028
**Market Players**
The major players covered in the medical devices vigilance market report are AB-Cube, AssurX, Inc., Oracle, Sarjen Systems Pvt. Ltd., Sparta Systems, Inc., Xybion Corporation, ZEINCRO Group, Arena Solutions, Inc., Intel Corporation, mdiConsultants, Inc., EXTEDO, Freyr, Greenlight Guru, Jama Software, RELX Group plc, MasterControl, Inc., Panacea Pharma Projects Limited, Smithers, Laerdal Medical, Qvigilance, among other domestic and global players. Market share data is available for Global, North America, Europe, Asia-Pacific (APAC), Middle East and Africa (MEA), and South America separately. DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
The global medical devices vigilance market is experiencing significant growth and transformation, with various segments such as delivery mode, application, end-user, and country contributing to the market dynamics. The evolution of on-demand/cloud-based delivery mode and on-premises delivery mode is reshaping how vigilance practices are implemented in the industry, offering flexibility and scalability for healthcare organizations. Application segments focusing on diagnostic, therapeutic, surgical, and research activities highlight the diverse uses of medical devices that require vigilant monitoring and risk assessment to ensure patient safety and regulatory compliance. Additionally, end-users such as CROs, OEMs, and BPOs play essential roles in driving the demand for advanced vigilance solutions to enhance healthcare quality and device safety across different regions.
Market players like AB-Cube, AssurX, Inc., Oracle, and other key industry participants are leveraging their expertise in vigilance solutions to meet the evolving needs of healthcare providers and regulatory bodies. These companies are investing in innovative technologies, strategic partnerships, and regulatory compliance to maintain a competitive edge in the market and drive sustainable growth. With a focus on market expansion and market share dominance across global regions, these players are at the forefront of shaping the future of medical devices vigilance through comprehensive solutions that address emerging challenges in the industry landscape. The competitive landscape analysis provided by DBMR offers valuable insights into the strengths and strategies of each market player, highlighting their contributions to market growth and innovation in vigilance practices.
In conclusion, the medical devices vigilance market is poised for continuous advancement and market expansion driven by the commitment of key industry players to patient safety, quality, and regulatory compliance. The emphasis on collaboration, innovation, and vigilance best practices will be crucial in sustaining growth and driving excellence in medical device safety and regulatory compliance efforts worldwide. As the market evolves, players will need to adapt to changing regulatory environments, technological advancements, and patient demands to maintain their competitiveness and contribute to the overall enhancement of healthcare delivery and patient outcomes globally.
The market is highly fragmented, with a mix of global and regional players competing for market share. To Learn More About the Global Trends Impacting the Future of Top 10 Companies in Medical Devices Vigilance Market : https://www.databridgemarketresearch.com/reports/global-medical-devices-vigilance-market/companies
Key Questions Answered by the Global Medical Devices Vigilance Market Report:
- What is the current state of the Medical Devices Vigilance Market, and how has it evolved?
- What are the key drivers behind the growth of the Medical Devices Vigilance Market?
- What challenges and barriers do businesses in the Medical Devices Vigilance Market face?
- How are technological innovations impacting the Medical Devices Vigilance Market?
- What emerging trends and opportunities should businesses be aware of in the Medical Devices Vigilance Market?
Browse More Reports:
https://www.databridgemarketresearch.com/reports/global-mineral-oil-market
https://www.databridgemarketresearch.com/reports/global-meat-starter-culture-market
https://www.databridgemarketresearch.com/reports/global-three-dimensional-conformal-radiation-therapy-market
https://www.databridgemarketresearch.com/reports/global-gonorrhea-testing-market
https://www.databridgemarketresearch.com/reports/global-capillary-electrophoresis-market
Data Bridge Market Research:
☎ Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 1011
✉ Email: [email protected]" Report this page